Curious about what schedule drug is buprenorphine? Look no further as we delve into the intricacies of this widely used medication. Buprenorphine, primarily known for its role in treating opioid addiction, falls under the category of Schedule III controlled substances. This classification indicates that while it has a potential for moderate to low physical dependence or high psychological dependence, it also offers accepted medical uses. Understanding the scheduling of buprenorphine is crucial for healthcare providers, patients, and individuals involved in substance abuse treatment. Join us on this enlightening journey to uncover the truth behind the scheduling of buprenorphine and its significance in the realm of addiction management.
Introduction to Buprenorphine
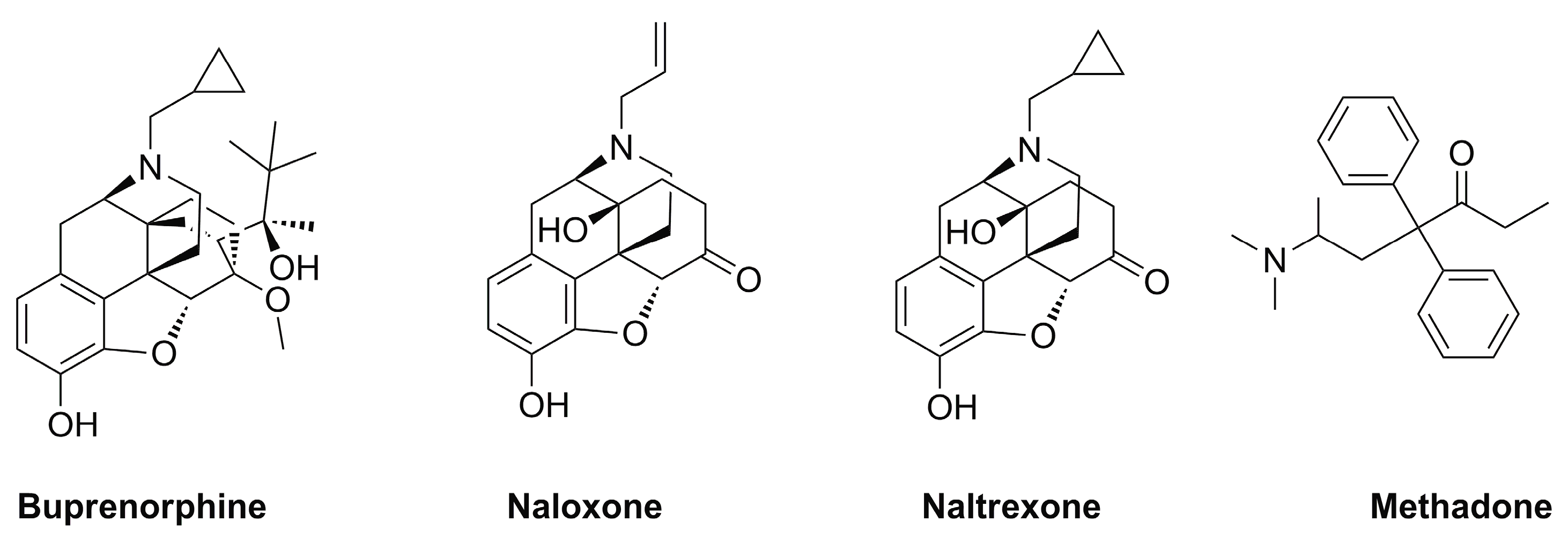
Buprenorphine is a medication primarily used to treat opioid addiction. It belongs to the class of drugs known as partial opioid agonists, which means it produces effects similar to opioids but with less intensity. Buprenorphine is commonly prescribed in combination with naloxone as a treatment for opioid dependence.
History of Buprenorphine
Originally developed in the late 1960s, buprenorphine has since gained recognition as an effective treatment for opioid addiction. Its unique pharmacology allows it to reduce withdrawal symptoms and cravings without producing the same euphoric effects as full opioid agonists.
Buprenorphine was approved by the Food and Drug Administration (FDA) in 2002 for the treatment of opioid dependence, marking a significant milestone in medication-assisted therapy for addiction.
Mechanism of Action
Buprenorphine exerts its effects by binding to opioid receptors in the brain, thereby blocking the effects of other opioids and reducing cravings. It has a long duration of action, allowing for once-daily dosing in most cases.
This unique mechanism makes buprenorphine a valuable tool in the treatment of opioid addiction by helping individuals manage their withdrawal symptoms and reduce the risk of relapse.
Understanding Drug Scheduling
Drug scheduling is a classification system that the government uses to regulate and control the manufacturing, distribution, and dispensing of certain medications based on their potential for abuse, medical use, and safety. Buprenorphine, a medication commonly used in the treatment of opioid addiction, is classified under this system.
Overview of Drug Schedules
The Drug Enforcement Administration (DEA) categorizes drugs into five schedules, with Schedule I being the most restrictive and Schedule V being the least. This classification is based on the drug’s medical value, potential for abuse, and safety concerns.
Drugs listed under Schedule I are considered to have a high potential for abuse and no accepted medical use, while those in Schedule V have a lower potential for abuse and accepted medical utility.
Buprenorphine’s Schedule Classification
Buprenorphine is classified as a Schedule III controlled substance under the Controlled Substances Act. This means that it has a moderate to low potential for physical and psychological dependence compared to drugs in Schedules I and II.
As a Schedule III drug, buprenorphine is recognized for its accepted medical use in the treatment of opioid dependence while still being subject to certain regulatory restrictions to prevent misuse.
Buprenorphine’s Classification
Buprenorphine, a medication primarily used to treat opioid addiction, falls under the classification of a Schedule III controlled substance in the United States.
Understanding Buprenorphine’s Classification
Buprenorphine is classified as a Schedule III controlled substance by the Drug Enforcement Administration (DEA). This classification indicates that while it has a potential for abuse, it is considered to have a lower abuse potential compared to drugs classified under Schedule I and II.
Moreover, buprenorphine has an accepted medical use in the treatment of opioid addiction and can be prescribed by healthcare providers who have obtained the necessary training and certification.
Regulations Surrounding Buprenorphine
Given its status as a Schedule III drug, buprenorphine is subject to specific regulations regarding its prescription, distribution, and use. These regulations aim to strike a balance between ensuring access to treatment for individuals struggling with opioid dependence and preventing its misuse or diversion for illicit purposes.
- Patient Monitoring: Healthcare providers are required to closely monitor patients receiving buprenorphine to track their progress, address any potential side effects, and prevent misuse.
- Prescription Guidelines: Providers must follow stringent guidelines when prescribing buprenorphine to ensure its safe and effective use in opioid dependence treatment.
- Storage and Handling: Facilities that dispense buprenorphine are expected to adhere to specific storage and handling protocols to prevent unauthorized access and ensure product integrity.
Implications of Buprenorphine’s Schedule
Buprenorphine is classified as a Schedule III controlled substance in the United States under the Controlled Substances Act. This scheduling indicates that while buprenorphine has a potential for abuse, it is considered to be at a moderate to low risk compared to drugs in Schedules I and II. The implications of buprenorphine being a Schedule III drug have significant impacts on its regulation, prescription, and usage.
Regulation and Prescription Requirements
As a Schedule III drug, buprenorphine is subject to tighter regulations compared to non-controlled substances. Healthcare providers must adhere to specific prescription requirements, such as limitations on refills and prescribing practices to prevent misuse and diversion.
Impact on Access to Treatment
The scheduling of buprenorphine also affects access to treatment for individuals with opioid use disorder. While the Schedule III classification allows for outpatient prescription of buprenorphine by qualified healthcare providers, there are still barriers to its widespread availability and affordability, which can hinder individuals seeking addiction treatment.
Benefits of Buprenorphine’s Classification
Buprenorphine is classified as a Schedule III controlled substance in the United States. This classification offers several benefits both for medical professionals and patients seeking treatment for opioid addiction.
1. Controlled Access
Being categorized as a Schedule III drug means that buprenorphine has less potential for abuse compared to drugs in higher schedules like Schedule II. This allows for controlled access to the medication, ensuring that it is available for those who truly need it for opioid dependence treatment.
2. Increased Treatment Availability
Due to its classification, buprenorphine can be prescribed by qualified healthcare providers outside traditional opioid treatment programs. This expanded access increases the availability of evidence-based medication-assisted treatment (MAT) for individuals battling opioid use disorder.
Frequently Asked Questions
- What is buprenorphine?
- Buprenorphine is a medication that is used to treat opioid addiction by reducing withdrawal symptoms and cravings.
- What schedule drug is buprenorphine?
- Buprenorphine is classified as a Schedule III controlled substance in the United States.
- How is buprenorphine administered?
- Buprenorphine can be administered sublingually (under the tongue), as a buccal film, or as an injection for certain formulations.
- Is buprenorphine safe to use?
- When used as prescribed and under medical supervision, buprenorphine is considered safe and effective for treating opioid dependence.
- Are there any side effects associated with buprenorphine?
- Common side effects of buprenorphine may include nausea, headache, constipation, and sweating. Serious side effects are rare but can occur.
Unraveling the Mystery: Understanding the Schedule of Buprenorphine
In conclusion, the classification of buprenorphine as a Schedule III controlled substance sheds light on the regulatory framework surrounding its usage. Its potential for abuse, albeit lower than Schedule I and II drugs, necessitates careful monitoring and regulation. Understanding the scheduling of buprenorphine underlines the importance of its safe and supervised administration in combating opioid addiction and managing pain. By recognizing buprenorphine’s schedule status, healthcare professionals and patients alike can navigate its use responsibly and effectively. Stay informed, stay vigilant, and advocate for responsible buprenorphine utilization to contribute to a healthier society.
